‘Junk’ DNA Could Be Recruited to Destroy Cancer Cells From Within
Sections of DNA once dismissed as dormant and useless could in fact be recruited to fight certain types of drug-resistant blood cancers, new research has revealed.
Known as ‘junk’ DNA, these bits of DNA don’t encode proteins, so were historically dismissed as having no role in biological processes. Scientists have since realized non-coding parts of the genome are more important than we thought, playing critical roles in gene regulation.
One category of non-coding DNA is the transposable element (TE): sequences that can cut themselves free from one part of the genome and insert themselves into another.
Related: Mitochondria Dump Their Rubbish DNA, And It Could Be Costing Us Our Health
An international team led by researchers from King’s College London (KCL) has now found that stubbornly persistent blood cancers can ‘wake up’ TEs as part of the mechanisms that lead to cancer cells going awry – and that this same activity could potentially be targeted to shut the cancers down.
More research is needed to validate these findings, which come from experiments with lab-grown cells, but unlocking a new pathway to target blood cancers could lead to treatments for cancers harboring specific mutations.
“This discovery offers new hope for patients with hard-to-treat cancers, by using existing drugs in a completely new way, turning what was once thought to be useless DNA into a powerful target for treatment,” says biologist Chi Wai Eric So, from KCL.
The research focuses on two blood cancers, myelodysplastic syndrome and chronic lymphocytic leukemia. Mutations typically found in these cancers damage the genes ASXL1 and EXH2, affecting protein production and leading to uncontrolled cell growth. A cascade of instability then follows.
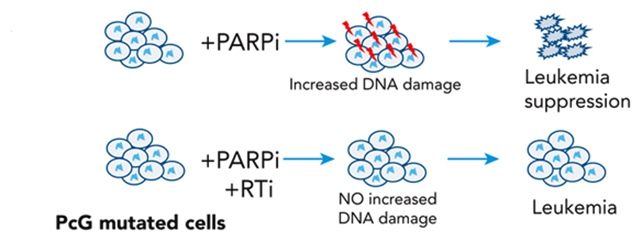
What makes targeting these cancers particularly tricky is that the mutated genes no longer produce proteins that conventional cancer therapies would target.
Using mouse models of cancer and human cancer cells, the researchers found another knock-on effect of ASXL1 and EXH2 damage: reactivated junk DNA duplicating and spreading by inserting their sequences throughout the cancer cells’ DNA.
This unfettered behavior stresses the cancer cells. To resist that stress and continue growing, the cancer becomes reliant on poly (ADP-ribose) polymerase repair proteins – or PARPs for short. Drugs that suppress PARPs were shown to be effective at killing off the two tested blood cancers. What’s more, healthy cells were mostly left alone.
“This study sets the stage for a novel and broader approach of creating synthetic lethality for human cancers,” write the researchers in their published paper.
The researchers are confident their findings apply to other types of cancers as well, not least because PARP blockers are already used to fight other forms of cancer, although the exact mechanisms involved differ.
And it’s another example of the hidden activity of TEs, previously dismissed as unimportant. Recent studies have found these DNA regions – which make up a large portion of the genome – help regulate the body’s defenses, aid the brain in dealing with fear, and even protect species from interbreeding.
“Transposable elements that account for almost half of the human genome, but are historically regarded as ancient junk sequences, have been reported in recent years to be reactivated in driving disease development, and multiple cellular processes including gene expression, DNA damage, and immune responses,” write the researchers.
The research has been published in Blood.
What did you think of this news? Leave a comment below and/or share it on your social media. This way, we can inform more people about the hottest things in technology, science, innovation, and gaming!
This news was originally published in:
Original source

